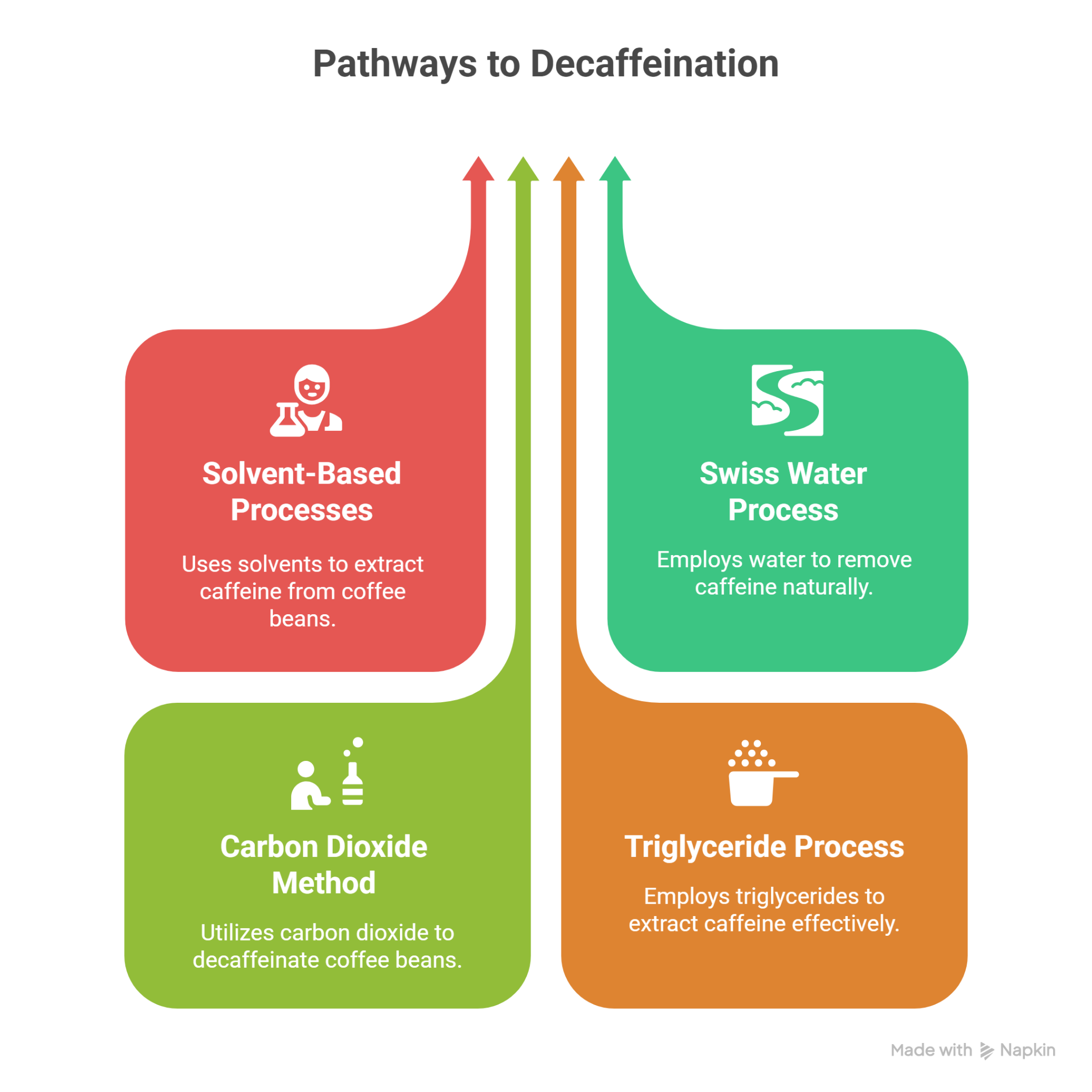
Coffee can be decaffeinated through several processes that remove most of the caffeine from green, unroasted beans before roasting. The most widely used methods are solvent-based (direct and indirect), Swiss Water, carbon dioxide, and triglyceride.
Solvent-Based Processes
There are two different types of solvent-based decaffeination processes, direct and indirect.
Direct
The direct-solvent process for decaffeinating coffee involves first steaming the green, unroasted coffee beans for about 30 minutes. This steaming opens up the pores of the beans, making the caffeine molecules more accessible.
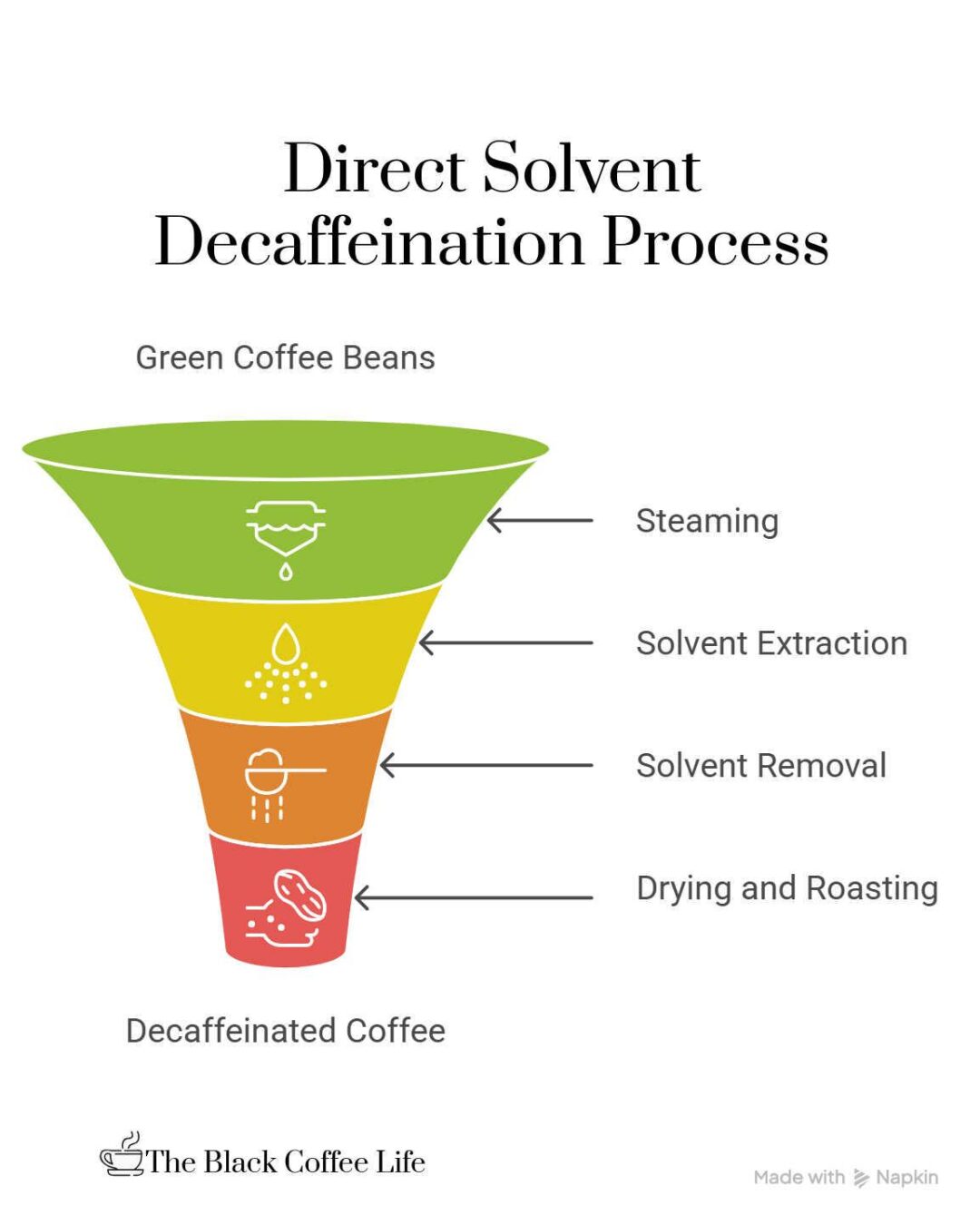
After steaming, the beans are soaked or rinsed repeatedly in a chemical solvent, typically methylene chloride or ethyl acetate, for approximately 10 hours. These solvents selectively dissolve and extract the caffeine from the beans. Once the caffeine has been removed, the solvent is drained away, and the beans are steamed again for several hours to remove any residual solvent.
Finally, the beans are dried and roasted as usual. This method directly exposes the beans to the solvent, which distinguishes it from other methods and helps it be relatively inexpensive and widely used. Although the process involves chemical solvents, the steaming and roasting phases remove nearly all residues, ensuring the resulting coffee is safe to consume.
Indirect
In contrast, the indirect-solvent process minimizes direct contact between the coffee beans and the chemical solvent. Initially, green coffee beans are soaked or steamed in hot water for several hours. During this soaking, caffeine and many flavor compounds dissolve into the water. Afterward, the beans are removed from the water, which is now rich in both caffeine and coffee flavor compounds.
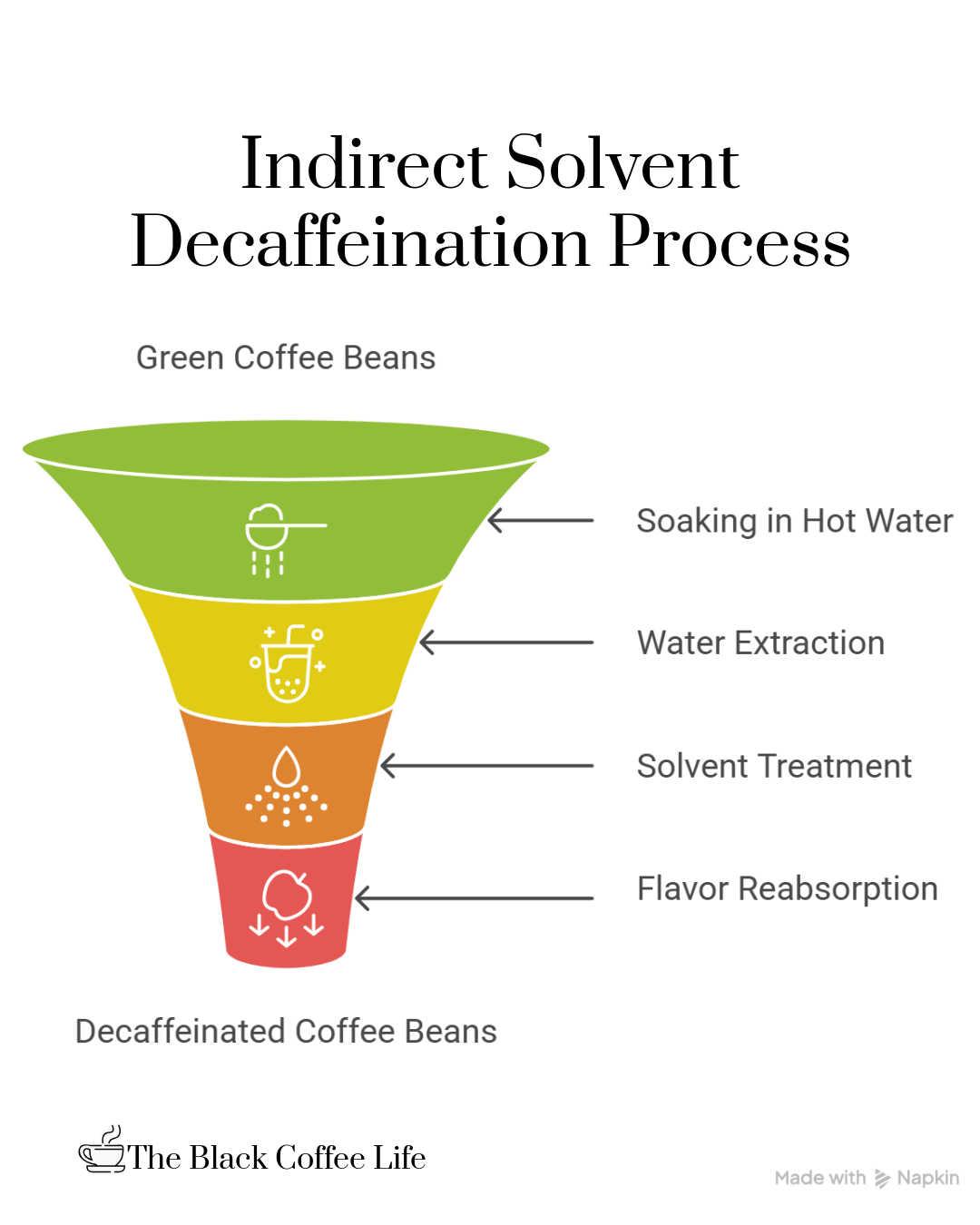
This extracted water is then transferred to a separate tank and treated with a chemical solvent, such as methylene chloride or ethyl acetate, to selectively remove only the caffeine molecules. The solvent is then separated from the water, leaving behind a caffeine-free but flavor-rich solution. This solution is returned to the beans, so they reabsorb the flavorful compounds while remaining caffeine-depleted.
Following this step, the beans are dried and roasted as usual. Because the solvent never directly touches the beans, this method helps better preserve the coffee’s original flavor compared to the direct-solvent process.
Swiss Water
The Swiss Water Process is a patented, natural, chemical-free method of decaffeinating coffee that relies on water and specialized filtration to remove caffeine while preserving the bean’s original flavor. The process begins with soaking green coffee beans in hot water, which extracts not only caffeine but also the coffee’s flavor compounds and oils into the water. The beans used in this initial soaking are discarded since both caffeine and flavor are lost from them.
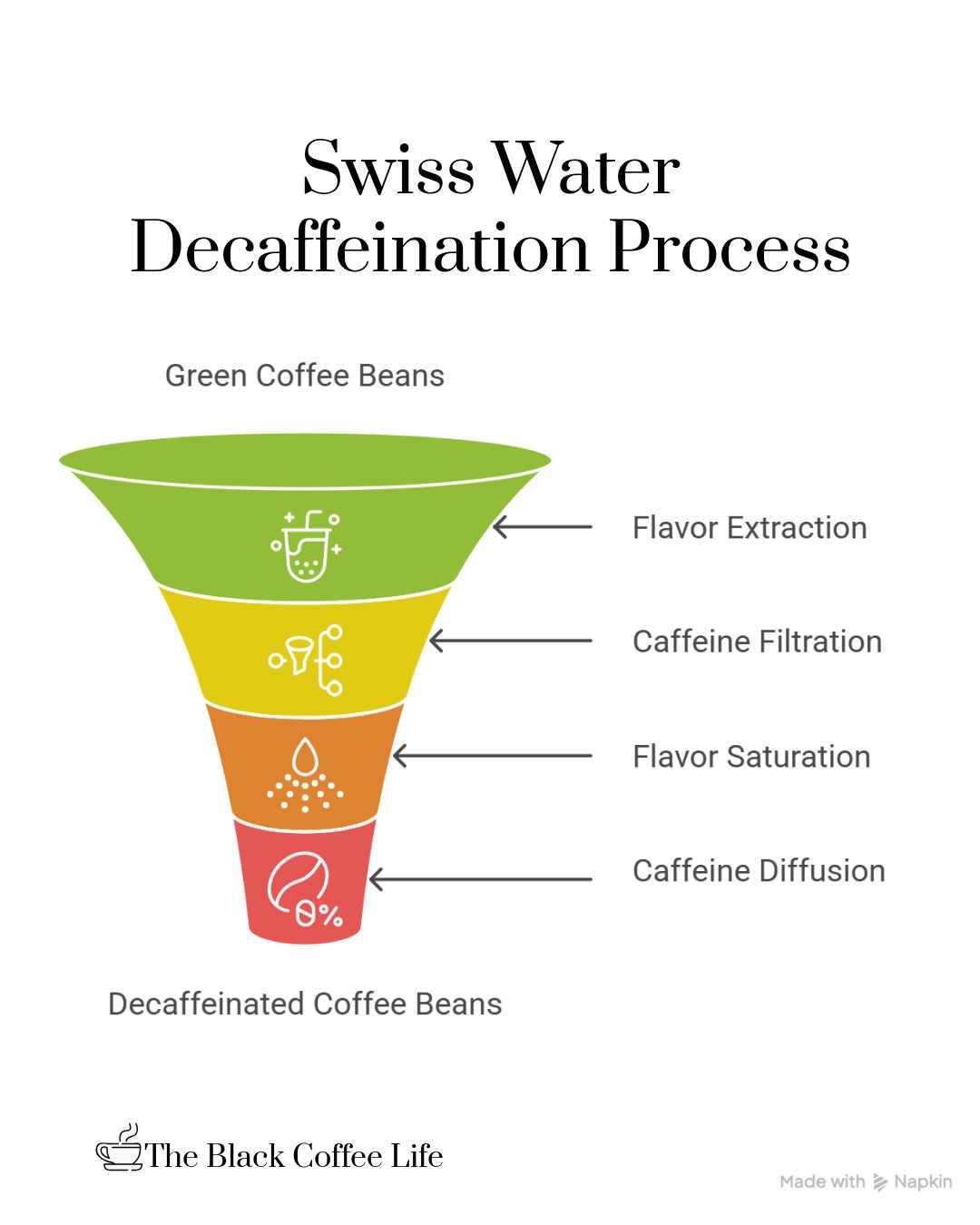
The water containing caffeine and flavor compounds is then passed through a proprietary carbon filter system. This system traps and removes the caffeine molecules but allows the flavor compounds to remain in the water. The result is a solution called Green Coffee Extract (GCE), which contains all the soluble flavors of coffee except for caffeine.
New batches of green beans are then soaked in this GCE solution. Because the GCE is already saturated with coffee flavor compounds, it prevents the beans from losing their flavor during soaking. Meanwhile, caffeine diffuses out of the beans into the solution due to the concentration difference. The caffeine-rich GCE is cycled through the carbon filters continuously to remove the caffeine, and this cycle repeats for several hours until the coffee beans are about 99.9% caffeine-free.
Once decaffeinated, the beans are dried and prepared for roasting. The entire process avoids the use of chemical solvents, making it an environmentally friendly and health-conscious option. Because the flavor compounds remain largely intact, coffee processed this way retains a taste very close to regular, caffeinated coffee. The Swiss Water Process is considered one of the best methods to produce high-quality decaf coffee with a full-bodied, rich flavor.
Carbon Dioxide
The Carbon Dioxide (CO₂) process for decaffeinating coffee is a sophisticated and natural method that uses liquid carbon dioxide under high pressure to selectively remove caffeine from green coffee beans while preserving the beans’ original flavors. It begins by soaking the green, unroasted coffee beans in water, which causes the beans to swell and allows the caffeine to dissolve into the water inside the bean. These water-moistened beans are then placed inside a large, sealed stainless steel extraction vessel.
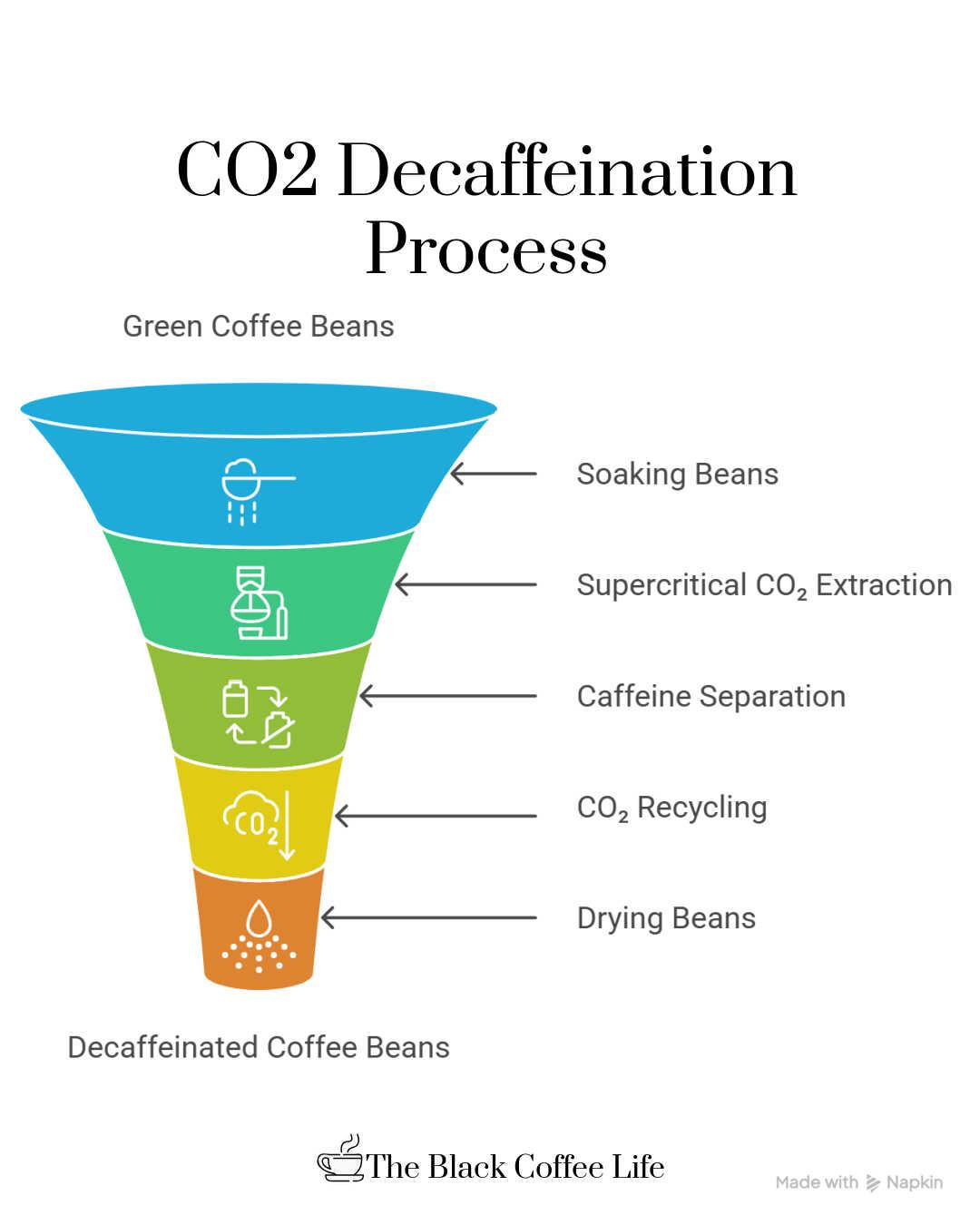
Next, liquid CO₂ is pumped into the vessel at very high pressure—about 1,000 pounds per square inch—and elevated temperature, reaching what is called the supercritical state where CO₂ exhibits characteristics of both a liquid and a gas. In this supercritical form, the CO₂ acts as a selective solvent that penetrates the coffee beans and bonds specifically with the caffeine molecules, extracting them efficiently but leaving most of the other flavor and aromatic compounds intact.
After allowing sufficient time—generally around 5 to 7 hours—for the caffeine to diffuse out, the caffeine-rich CO₂ is transferred from the extraction vessel to a different chamber where the pressure is reduced, or water is introduced, causing the CO₂ to release the caffeine. This freshly separated caffeine is collected and often sold for other commercial uses, such as in pharmaceuticals or soft drinks. The purified CO₂ is then recycled and pumped back into the extraction vessel to continue the decaffeination cycle.
Once the caffeine has been removed, the beans are gently dried to restore their optimal moisture content before they are roasted and packaged for sale. Because the CO₂ method targets caffeine very selectively, it preserves much of the coffee’s original flavor profile and aroma better than many other decaffeination processes. Additionally, it is considered environmentally friendly due to the recycling of CO₂ and the absence of chemical solvents, making it a popular choice for producing high-quality, naturally decaffeinated coffee.
Triglyceride Process
The triglyceride process is a relatively newer and less common method of decaffeinating coffee that uses natural coffee oils, specifically triglycerides extracted from spent coffee grounds, to selectively remove caffeine from the beans. The process begins with soaking green coffee beans in a hot water and coffee solution. This soaking causes the caffeine, along with some flavor compounds, to migrate toward the surface of the beans.
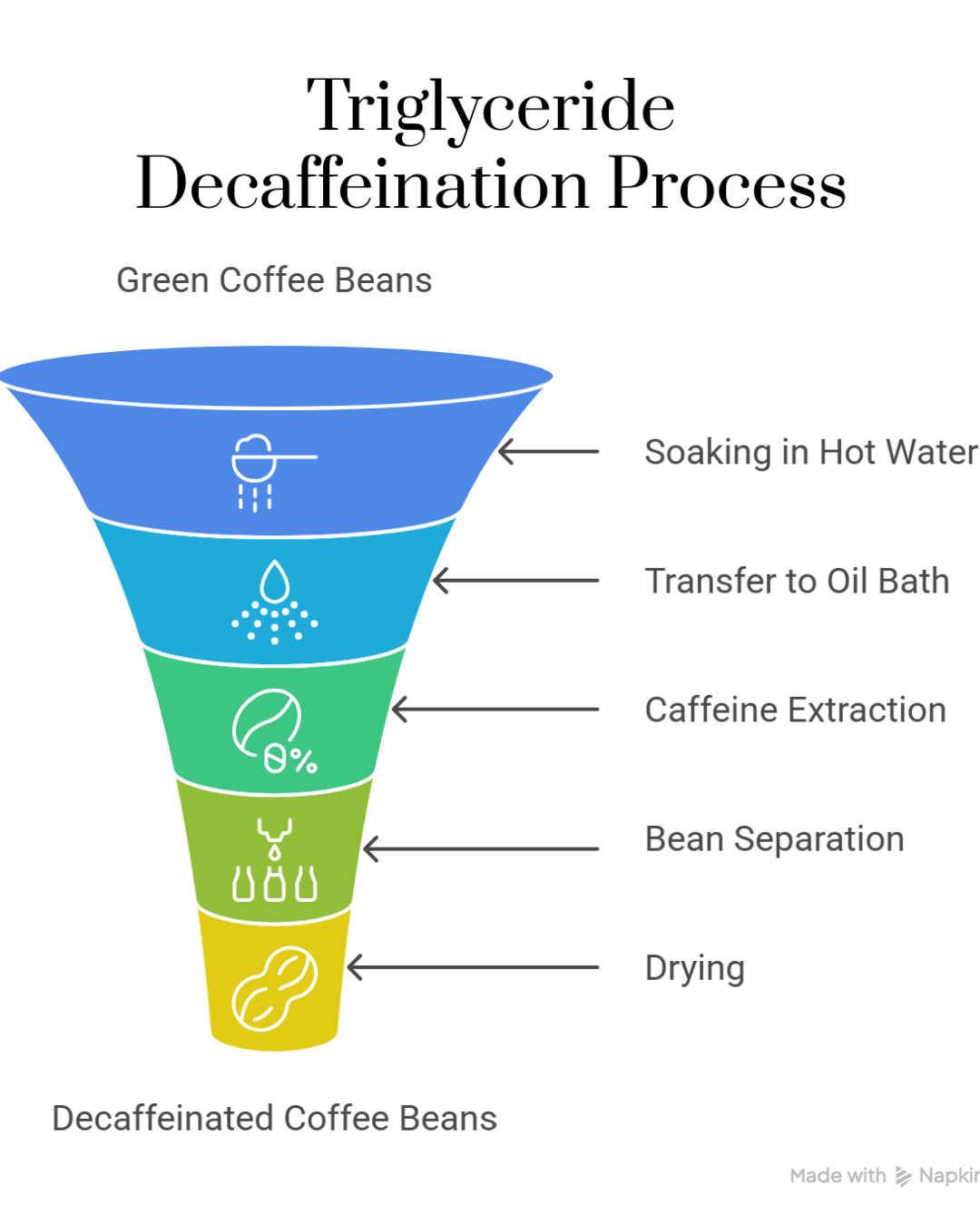
After this, the beans are transferred to a separate container where they are immersed in coffee oils derived from previously used coffee grounds. These oils contain triglycerides, which are fatty acids that selectively bind to and extract caffeine molecules from the surface of the beans. The beans are left in this oil bath for several hours under elevated temperatures, allowing the triglycerides to pull out the caffeine while leaving the flavor compounds largely intact.
Once the caffeine is absorbed by the coffee oils, the beans are separated from the oils and dried, completing the decaffeination process. The caffeine-laden oils can be treated and recycled for use in subsequent batches, making the process more sustainable. Because this method avoids synthetic chemical solvents, it is attractive to consumers seeking a more natural, solvent-free decaf coffee. Additionally, this process is effective at maintaining the original flavor and aroma of the coffee beans, resulting in a decaf coffee that closely resembles its caffeinated counterpart in taste. The triglyceride process is typically used for specialty and organic beans and is considered a premium, environmentally friendly decaffeination option.
Decaf Coffee and Caffeine Content
Regardless of method, decaf coffee is not 100% caffeine-free. Typically, about 97% of caffeine is removed. A cup of decaf contains only a small fraction of the caffeine found in regular coffee—you would need to drink 10+ cups of decaf to equal one caffeinated cup.
Is Decaffeinated Coffee Safe?
All current decaffeination methods are considered safe by global food regulators. Solvent residues in beans are minimal and eliminated during roasting.
For most people, the overall health differences between decaf and regular coffee are likely small. However, those concerned about possible chemical exposure or with specific sensitivities may prefer water-processed or CO₂-processed decaf.
Which Of The Methods Produce A Flavor That Is Closest To Caffeinated Coffee?
For decaf coffee flavor most similar to caffeinated coffee, look for CO₂ Process or Swiss Water Process decaf. Decaffeination processes often reduce some volatile aroma compounds like pyrazines, which contribute to nutty, roasted, and chocolate notes in coffee. Solvent-based methods generally alter flavor more noticeably but can still produce good-quality decaf.
Summing Up
Each method of decaffeinating coffee is a complex process that balances the removal of caffeine with the preservation of flavor, and offers distinct advantages and potential trade-offs. Whether prioritizing natural, chemical-free approaches or aiming for the closest flavor to regular coffee, there are options to suit every preference and concern. Understanding these methods not only deepens appreciation for your cup of decaf but also empowers you to make informed choices about the coffee you enjoy.
Thanks for the informative article. I don’t drink decaf, but I may have to some day. I’ve tried it before and don’t think there was a significant difference in taste.